Last Updated on September 5, 2022
Overview:
Clinical Trials act as a roadmap for the Research Industry by providing thorough information on new drugs, medical devices, or medical strategies. Clinical Trials are an integral part of the drug development and marketing process. However, certain roadblocks impede the efficiency of Clinical Trials. One of these is the lack of diversity in clinical trial recruitment.
In this blog, we will go through the process of patient recruitment, the role of Clinical Trial diversity, the FDA’s stance on diversity and inclusivity, and a lot more.
What is Diversity?
Diversity refers to empowering people by respecting and accepting their differences, including those related to age, gender, race, religion, disability, sexual orientation, and country of origin. The reflection of these distinctions is possible by diversity in a supportive, encouraging, and safe atmosphere. It enables us to value diversity in the community and the workplace while also embracing and celebrating the rich diversity that each individual possesses.
Clinical Trial Diversity: Statistics
According to WHO statistics, the percentage of clinical trials is divided into various categories, including:
A- Trials per year – Worldwide:
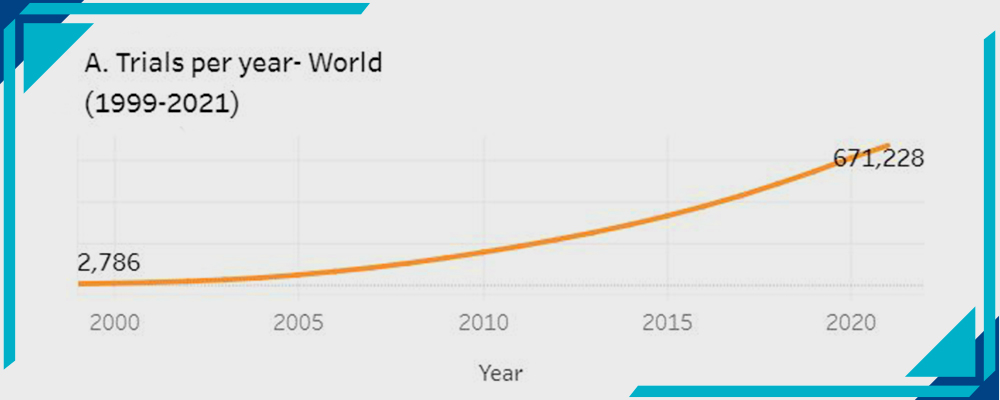
Statistics by WHO
The number of trials conducted from the year 2000 to 2022 has significantly increased. It was 2786 back in 2000 and it raised to 671,288.
B- Trials by Phase of Development:
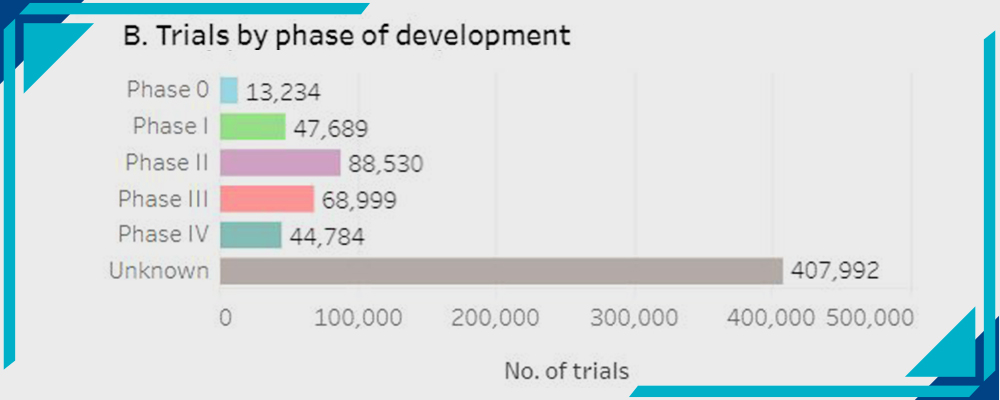
Statistics by WHO
- Phase 0: 13234
- Phase 1: 47,689
- Phase 2: 88,530
- Phase 3: 68,999
- Phase 4: 44,7854
C- Trials by Year and WHO Region:
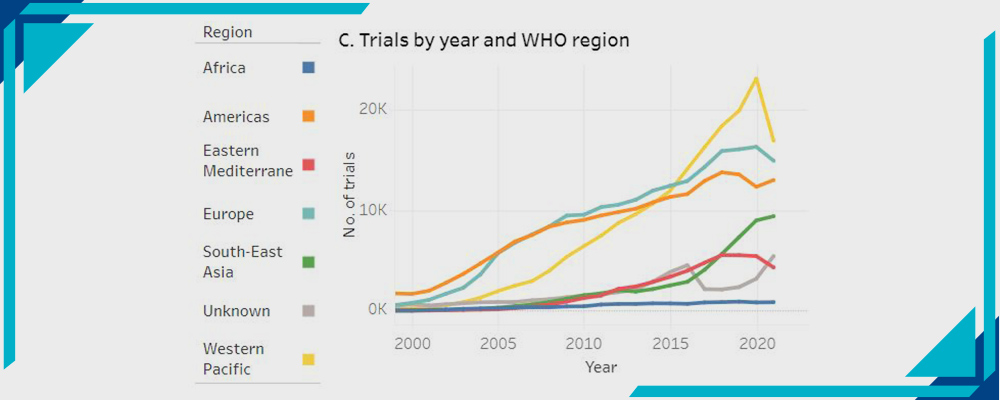
Statistics by WHO
- Africa
- America
- Eastern Mediterranean
- Europe
- South East Asia
- Western Pacific
- Unknown
D- Trials by Health Category:
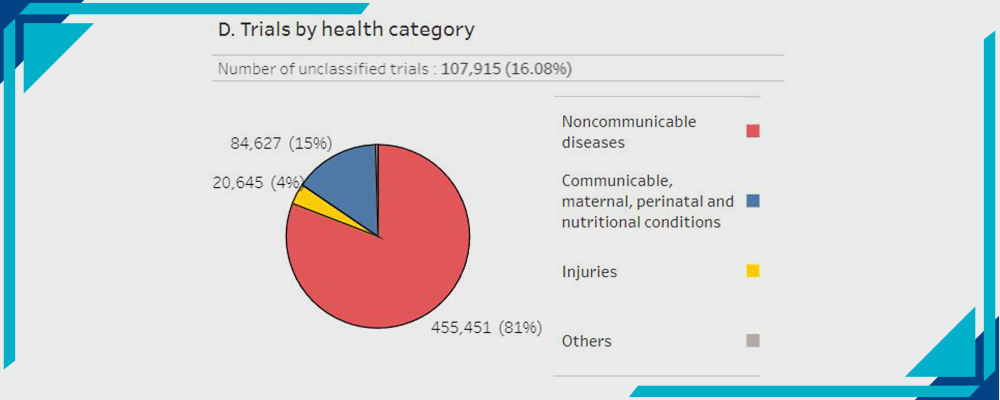
Statistics by WHO
15% Communicable, maternal, perinatal, and nutritional conditions.
E- Trials by Country or Area:
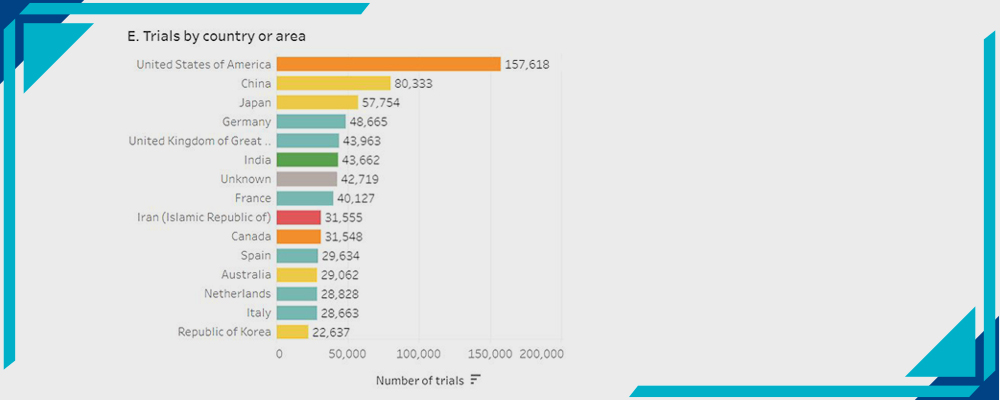
Statistics by WHO
The USA has the highest rate of Clinical Trials with 157,618 trials.
F- Trials by Sex of Participants:
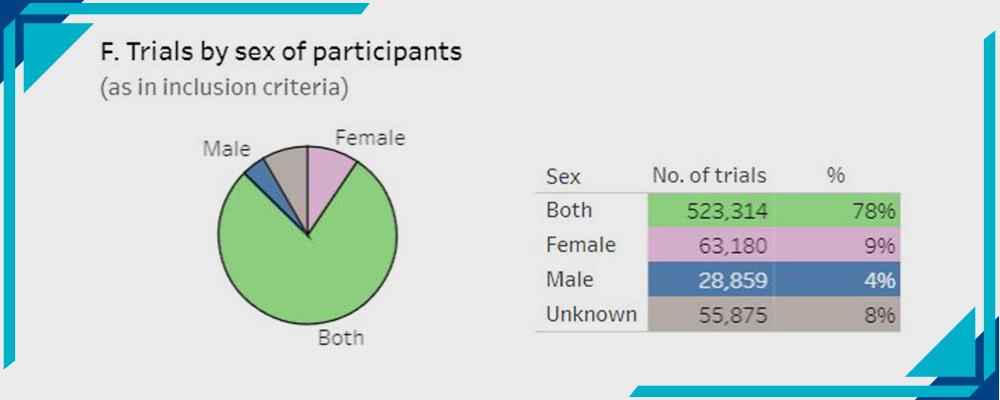
Statistics by WHO
- Both 78%
- Females 9%
- Males 4%
- Unknown 8%
G- Trials by Age:
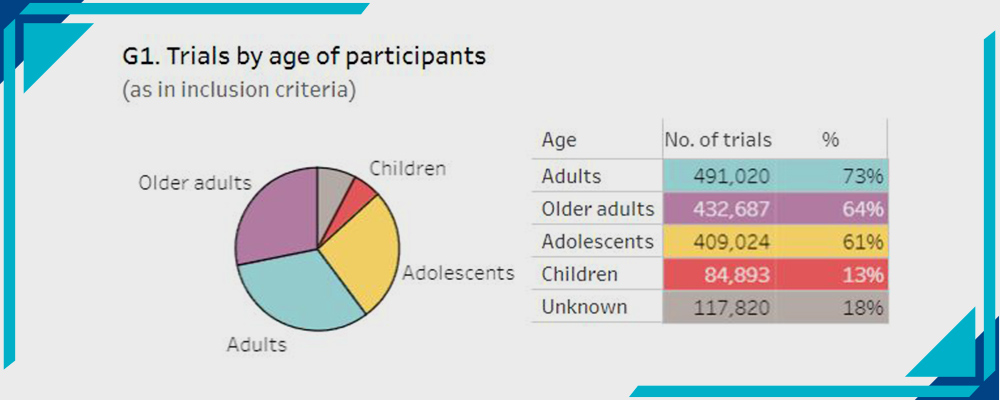
Statistics by WHO
- Adults 73%
- Older adults 64%
- Adolescents 61%
- Children 13%
- Unknown 18%
What Does Clinical Trial Diversity Denote?
Diversity in Clinical Trials represents a mix of people who volunteer in a Clinical Trial and are tested for a new drug or medical device. These people may have a different reaction to the same drug, or device depending on various factors, like age, gender, weight, race, and ethnicity.
It is therefore essential to have people from different backgrounds participating in different ethnicity clinical trials as it eliminates biases, promotes health equity and social justice along with producing more innovation in the research industry.
Diversity in Clinical Trial Recruitment:
During the Clinical Trial patient recruitment process, sponsors, sites, or recruiting firms look for ways to connect with people who might be eligible for their trial. It’s a multi-step procedure that starts with detailed patient population research and the development of an individualized outreach plan, increasing inclusivity and diversity in the clinical trial recruitment process. Having a diverse sample is crucial to the success of a clinical trial because it aids in assessing if the adverse reaction is a result of the drug or medical device before it is marketed.
Methods that are usually adopted to achieve diversity in clinical trial recruitment include:
- Digital advertising,
- Partnerships,
- Campaigns,
- Print media, and
- Website.
Diversity of the Target Population:
The diversity of the target population should be graphically reflected in the campaign materials. Involving communities of color in the development of recruitment techniques and materials to make sure marketing campaigns resonate with a variety of audiences is the key to successful clinical trials. With imagery that reflects who they are and what is important to them, participants are more likely to connect. The graphics, language, and targeting of successful campaigns and persuasive advertising must reflect the actual patient population.
Additionally, recruitment materials should include participants’ feedback and reflect the opinions that participants are expressing to one another. Clinical trial diversity requires ensuring equality. Everyone should be provided with the opportunity to speak up, take part, and help those in their communities. Deciding to take part in a clinical trial is a personal decision that should be discussed with your doctor and your support system, which includes family and friends.
What are the Challenges in Clinical Trial Diversity?
Minorities rarely participate in trials for a variety of reasons, several of which are recognized as:
- Lack of trust in the research community,
- Logistical and time restrictions, and
- A lack of awareness about clinical trials.
These are the top three obstacles Sponsors and CROs face when trying to incorporate diversity in clinical trial recruitment.
A variety of other barriers to clinical trial diversity are identified based on various factors. Some of the obstacles include a lack of clinical investigators from marginalized groups, mistrust of the clinical research, geographic and economic factors that deter communities of color from participating, and a lack of inclusion of diverse communities in the planning and execution of research or ethnicity clinical trials.
Absence of comprehensive and trustworthy data:
The absence of comprehensive and trustworthy data on race and ethnicity in the computerized patient databases held by an expanding number of clinical providers is another hurdle that necessitates action on a government level. Such databases offer quick and effective ways to find persons who fit certain criteria in terms of their clinical characteristics, provided the patient gives their consent. This leads to clinical trial diversity and ethnicity clinical trials. Then, if they are interested in taking part in trials, they can be approached. If accurate race and ethnicity data are included and searchable in these databases, it would considerably aid the formation of diverse cohorts and push the agenda of ethnicity clinical trials.
Nonetheless, it will be challenging to overcome these barriers, but a variety of public policy initiatives would encourage the development of data sources from which different clinical trial groups could be more effectively and securely put together.
What is the Stance of the FDA on Clinical Trial Diversity?
Draft guidance titled “Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials” was released by the US Food and Drug Administration (FDA). The Guidance sets forth requirements for producers of FDA-regulated medical products to create a Race and Ethnicity Diversity Plan for recruiting a sufficient number of individuals from underrepresented racial and ethnic populations to participate in clinical trials of their products and increase diversity and ethnicity clinical trials.
The FDA promotes clinical trial enrollment for people of all backgrounds. As for clinical trials to advance health equality, participants must come from a variety of backgrounds. Patients who will use the medicinal commodities should be represented in clinical trials. However, it contradicts the usual practice, where individuals from varied racial and cultural backgrounds are marginalized in clinical research. This is concerning since various age groups, races, and ethnic groups may respond differently to particular medical items and it requires attention.
Role of Pharmaceuticals in Promoting Clinical Trial Diversity:
Increasing corporate responsibility to ensure interaction with underrepresented communities enhanced regulatory involvement to encourage corporations, and increased diversity awareness campaigns are just a few strategies to create change. As a sector, they have the power to make a significant difference. Making Clinical Trials more diverse leads to a better knowledge of a drug’s mechanism of action and increases a drug’s acceptability among patients, as the COVID-19 vaccination studies depicted.
COVID-19 led to a massive shift in the Clinical Trial process. As the world was hit by a deadly pandemic, it was the need of the hour to develop vaccines that catered to all communities of color. This resulted in the idea of diversity and inclusiveness in Clinical Trials. With the evolution of disease states, Pharmaceuticals are constantly in pursuit of incorporating the best practices to promote inclusiveness and clinical trial diversity.
Scope of Decentralized and Virtual Trials to Promote Clinical Trial Diversity:
Decentralized clinical trials can make research more accessible to people from a wider demographic who might not otherwise participate in site-based research. Improvements in participant recruitment and retention increased involvement and diversity, and a decrease in the expenses associated with conducting trials are additional advantages.
Innovative technologies and participant-centered design are combined in decentralized clinical trials. Decentralized clinical trials can be conducted entirely remotely or using a hybrid technique that requires some physical site attendance also. They are accomplished through the use of direct-to-patient drug delivery, home health professionals, neighborhood labs, digital consent, data gathering, and remote monitoring and diagnostics. These studies are designed to lessen the need for face-to-face interactions between researchers and participants and increase clinical trial diversity.
Decentralized Clinical Trials:
Decentralized Clinical Trials not only benefit the participants but also the sponsors by taking into account a variety of patient needs that are frequently overlooked in conventional designs.
As Decentralized Clinical Trials encourage underrepresented communities to take part for the betterment of their health, it brings diversity to clinical trial recruitment and increases inclusivity in ethnicity clinical trials. Besides, this can entail working with participants in their local communities through decentralized trials, but it can also encompass reconsidering exclusion standards, developing a diverse workforce, and developing trust between research personnel and participants resulting in clinical trial diversity.
Collection of Race and Ethnicity Data in Clinical Trials:
Medical and public health researchers suggest that the success of their study depends on the inclusion of race and ethnicity in clinical trials. In terms of the etiology of disease and the interaction between genetic and environmental factors, it is hypothesis-generating.
The interaction of socially mediated exposures and genetically determined elements leads to complex health outcomes. Race and ethnicity are routinely measured as possible predictor factors in medical and public health research because they are fundamental to both of these. Race and ethnicity data in Clinical Trials are the main predicting variables for an outcome in various research.
Challenges:
Disparities in the collection of race and ethnicity data in a Clinical Trial are extremely challenging to deal with and are present throughout all research studies. These disparities range from differences in the prevalence of diseases like thalassemia to differences in the prognosis of diseases like ovarian cancer to the unequal utilization of healthcare resources.
Since ethnic minorities are frequently underrepresented, it might be difficult to select representative samples with sufficient sample sizes.
How to Enhance Representation and Clinical Trial Diversity?
Every minority group has different demands and difficulties, which must be studied from the perspectives of public health and social justice. Diversity-centered health policies are the need of the hour. To promote health equity and improved health outcomes, we need to diversify the workforce to enhance diversity in clinical trial recruitment. In this way, we can promote inclusive ethnicity clinical trials and clinical trial diversity.
Factors:
Numerous factors, including preexisting health imbalances, access restrictions, and public mistrust of medical research. Contribute to the lack of ethnicity clinical trials and clinical trial diversity. The FDA has also talked about how accessibility issues to research sites and inclusion/exclusion criteria can contribute to a lack of diversity.
It is difficult to list every factor that could contribute to clinical trials not being diverse enough, but the four major aspects that can be broken down are:
- Lack of access to study sites,
- Lack of time and resources for study participation,
- Overly strict study inclusion criteria, and
- Patients do not feel like the researchers leading the trial are representing them.
While some of these issues can be resolved through technology, others call for a more extensive strategy. Some of the strategies to improve clinical trial diversity could be:
Improving Accessibility through Decentralized Trials:
Clinical trials may not be available to patients who reside in remote or inaccessible locations because the research site does not have enough means to contact them or because they are unable to travel to the site. As a result, the patient group that researchers can draw from is less diversified, meaning less ethnicity clinical trial and overall clinical trial diversity. The fact that decentralized clinical trials can be conducted anywhere can help overcome this issue.
While some decentralized studies allow participants to submit all of their data remotely via apps, telehealth visits, or wearable technology, others allow participants to visit local pharmacies and clinics.
Including Socioeconomic Considerations:
People do not just refuse to participate in clinical studies because of distance. Some people are unable to afford the concurrent medical expenses, while others are unable to take time off work or cover childcare fees.
Clinical trials must be reasonably priced or paid to be truly inclusive. Although technology providers and research centers can not cover all of a patient’s expenses, they can assist hourly employees and parents. If participants may check in while at work, at home, or both, more people will take part in Clinical Trials. Participants in underdeveloped or rural areas may benefit from being permitted to check in at the nearest pharmacies or medical offices. Providing easy access to participants will encourage them to participate and this will resultantly increase clinical trial diversity.
Reanalyzing Inclusion and Exclusion Criteria:
To conduct clinical trials that are secure and efficient, researchers must establish inclusion and exclusion criteria that portray inclusivity and diversity. However, to avoid excluding potential participants on faulty grounds, the FDA urges research sites, CROs, and sponsors to carefully consider how they set inclusion criteria so that clinical trial diversity is ensured.
Make Participants Feel Valued and Welcomed:
Some people don’t trust the medical research sector, and they might not want to take part in clinical studies. By allowing participants to consult with nearby healthcare providers they already have confidence in, decentralized trials might relieve this apprehension.
However, what if patients need to communicate with clinical research workers or don’t have access to a healthcare provider? By having a varied workforce, research sites can increase trust between participants and the research staff. If they have a one-on-one interaction with the trial’s administrators, patients may be more willing to take part in clinical research rendering clinical trial diversity.
Additional strategies:
Additional strategies to ensure the involvement of marginalized communities and to improve diversity in clinical trial recruitment are:
- Look into the specific challenges each minority population faces, then create a suitable health policy plan to address each challenge. The FDA should make diverse enrollment a top priority, and important professional associations should put policies in place to improve the participation of minorities in clinical trials.
- Recognize the crucial importance of socioeconomic determinants of health and how they impact the participation of minorities in clinical studies and ethnicity clinical trials. Effective mitigation measures should be created for the socioeconomic inequalities that disproportionately affect minority communities.
- With the assistance of neighborhood groups and community leaders, develop health literacy and awareness initiatives. Minorities receive fewer opportunities to get screening than the national average.
Aid minority populations:
Local organizations can aid minority populations in developing their health literacy in several ways, including the ones listed below:
- Local organizations like churches and community groups can utilize visual aids to describe the screening and other services offered in clinical trials.
- For cancer sufferers, establish social support groups both offline and online.
- Carnivals, fairs, and neighborhood gatherings can be used for enrollment and screening.
- Promoting awareness through activities on local radio stations.
- Enhancing clinical trial diversity by increasing the enrollment of minorities by broadening the clinical trial eligibility requirements. It is generally considered that minorities experience comorbid conditions more frequently. Consequently, more flexible eligibility requirements will increase minorities’ involvement and participation in the clinical trial process.
- Minority recruitment for clinical trials should begin within the first stages of trial design, i.e., Phase I. This will make it easier to spot early toxicity or efficacy cues. The public health continuum will benefit from better disease prediction and treatment choices as a result of the increased representation.
- Make contact with Faith-Based Organizations (FBOs), which could play a role in educating people about Clinical Trials. The absence of trust is a key problem in clinical trials. Organizations with a strong community and religious foundation can do a lot to build the required trust.
Taking above mentioned steps would surely foster clinical trial diversity and enhance ethnicity clinical trials.
Outlook:
Needless to say, diversity in Clinical Trials recruitment is the way forward in making medical advancements a possibility. Research progress and its dissemination depend largely on diversity, equality, and inclusivity. Lack of diversity leads to constraints on the prospect of Research. It lessens the potential of using science to help the world.
As difficult as it seems, including people from diverse backgrounds can lead to massive evolution in the Research sector. Diversity is an integral element in human existence. It enhances ideation and encourages the quest for novel information, leading to better decision-making. Diversity promotes unbridled discoveries and breakthrough inventions.
By adopting meaningful strategies to incorporate clinical trial diversity. Pharmaceuticals and Clinical Research Organizations can take Clinical Trials to another level. This will not only help the medical fraternity but will also help in building healthy communities.
Revive Research Institute is dedicated to conducting Clinical Trials inclusive of diversity. Our research staff is bi-lingual and we go above and beyond to ensure accessibility of Clinical Trials regardless of age, gender, race, ethnicity, or bias.




